Fluid Bed Incineration
Nooryusmiza Yusoff, Bonny Moy and Catherine Tong
December 9, 1996
Executive Summary
Fluid bed incinerator (FBI) has been widely used for the incineration of municipal, commercial and industrial waste since the early 1960's. Its simplicity of construction and flexibility in accepting any types of fuels, make the FBI very useful in industry. A study on a bench-scale FBI was performed to investigate the variations of pressure drop and temperature along the bed axis, and the effect of air-to-fuel ratio over the composition of the flue gas. In addition, it is also desired to observe how different types of waste samples affect the quality of the exhaust gas. Pressure drop and temperature were determined to increase with increasing bed height. The composition of the combustion products (CO2 and H2O) was found to increase with decreasing air-to-fuel ratio. The quality of the exhaust gas depended on both the chemical and physical properties of the waste samples. Although its construction is simple, the FBI must be operated with cautious. The bed temperature must be kept low and monitored continuously to prevent bed meltdown and the formation of air pollutants. Finally, if environmental issues are of a great concern, it is recommended that different methods of waste disposal be used instead of the incineration method.
Keywords
Fluid bed incineration, waste disposal, pressure drop, adiabatic flame temperature, flue gas composition, minimum fluidization velocity, Ergun equation, gas chromatograph
Table of Contents Page
Introduction 1
Summary 1
Recommendations 1
Discussion 2
References 8
Appendices
Figures
Tables
Introduction
Fluid bed incinerator (FBI) has been used for the incineration of municipal, commercial and industrial waste since the early 1960's because of its interesting properties; homogeneous operating temperature and good contact between solids and gases inside the bed are among the reasons why FBI is so popular. In addition, its simplicity of construction and flexibility in accepting solid, liquid and gaseous fuels makes the FBI very useful in industry. Recently, FBI is used in coal power plant to generate electricity because it has high combustion efficiency, even at low temperature. To obtain an insight on how the FBI works, a study on a bench-scale FBI was performed; the objectives are to investigate the variations of pressure drop and temperature along the bed axis, and the effect of air-to-fuel ratio over the composition of the flue gas. Additionally, it is also desired to qualitatively observe the exhaust gas when different types of waste samples are injected to and thrown from the top of the bed.
Summary
Recommendations
It is important to note that if the air and fuel are fed stoichiometrically, the flame temperature can reach as high as 3500 oF. This temperature is far above the melting temperature of the bed. Therefore, it is highly recommended that air is fed in excess to not only prevent bed meltdown but also to ensure complete combustion. Futhermore, at high temperature, there is a possibility of the formation of nitrogen oxides (NOx) and dioxins. Hence it is recommended that the operation temperature be maintained between 1400-1600 oF. To increase the residence time inside the FBI, the air velocity must be reduced to a value near the minimum fluidization velocity. To minimize the pressure drop variation, the bed height should be kept to the lowest possible. For the unsteady state operation, the place where the waste samples are injected or dropped was determined to be important. It is recommended that wastes are injected into the bed via injection port at the bottom of the bed; this is important to increase the residence time of the wastes inside the bed. In addition, both the chemical and physical properties of the waste samples were determined to be important because different wastes produce different types of fly-ash, smoke and flame. Finally, if environmental issues are of a great concern, different methods of waste disposal such as molten salt/metal technology and supercritical water oxidation may be used to replace the incineration method.
Discussion
A study on a fluid bed incinerator (FBI) was performed at laboratory scale. Before starting the operation of the FBI, minimum fluidization velocity (Umf) of air needs to be calculated. This is necessary to ensure that fluidization occurs during the combustion of methane and also to prevent the possibility of feeding too much air into the bed; high air velocity might cause the fluid-bed media (particle) to be blown out of the bed. The Umf can be found from Equation 1.
![]() Re
Re![]() 20 (1)
20 (1)
where Umf is the minimum fluidization velocity (ft/s), dp is the particle diameter (ft), rp is the particle density (lb/ft3), rg is the gas density (lb/ft3), m is the fluid viscosity (lb/ft.s), g is the gravitational constant (32.17 ft/s2) and Re is the Reynolds number (rgUmfdp/m).
The theoretical adiabatic flame temperature (Tad) was calculated at different methane flow rates (0.11 to 0.17 lbmole/min). Equation 2, obtained from the energy balance with no heat loss and mechanical work done, is used to calculate this value.
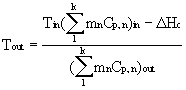 (2)
(2)
where Tout is the theoretical adiabatic flame temperature (oF), Tin is the temperature of the feeds (oF), mn is the molar flow rate of species n (lbmole/min), Cp,n is the heat capacity of species n (Btu/lbmole.oF) and DHc is the heat of combustion of methane (Btu/lbmole). The values of the experimental flame temperatures were taken directly from the thermocouples. Table 1 displays the comparison between the theoretical and experimental Tad.
Table 1: Theoretical and experimental adiabatic flame temperature
|
Run |
Theoretical Tad (oF) |
Experimental Tad (oF) |
|
1 |
1933 |
1699 |
|
2 |
2689 |
1794 |
|
3 |
2835 |
1289 |
The experimental Tad was found to be much lower than the theoretical Tad. On the first run, the difference is about 12%. On the second and third runs, the differences between the theoretical and experimental values are 33% and 55%, respectively. These results are expected because not only the top of the FBI was not covered but the bed was also not properly insulated. These two factors led to the conclusion that the initial assumption of an adiabatic bed was not valid. In order to obtain the correct theoretical flame temperature, heat loss must be taken into account in the energy balance.
The variation of pressure drop along the bed axis was investigated. The theoretical pressure drop was calculated by Ergun equation, shown in Equation 3.
![]() (3)
(3)
where DP is the pressure drop (lb/ft2), Em is the fraction void, m is the viscosity of air (lb/ft.s), Umf is the minimum fluidization velocity (ft/s), Fs is the sphericity (0.9 for this case), dp is the particle diameter (ft), rg is the gas density (lb/ft3), H is the height of the bed (ft) and gc is the gravitational constant (32.17 ft/s2). The experimental pressure drop was measured by the pressure drop meter and compared to the theoretical value in Table 2 below.
Table 2: Theoretical and experimental pressure drop, DP
|
Run |
Bed Height (in) |
Theoretical DP (in of water) |
Experimental DP (in of water) |
|
1 |
7.5 12.5 17.5 7.5 |
2.24 2.62 3.02 2.19 |
3.80 5.85 6.20 2.76 |
|
2 |
12.5 17.5 7.5 |
2.61 3.06 2.26 |
4.61 6.00 3.39 |
|
3 |
12.5 17.5 |
3.79 2.81 |
5.12 6.05 |
As expected, the theoretical DP differs from the experimental DP because the Ergun equation is not valid in the fluidized regime. On the first run, the difference ranges from 70% to 120%. On the second and the third runs, the differences range from 26% to 96% and 50% to 115%, respectively. In order to obtain a better understanding on how DP varies along the bed axis, a plot of DP vs. bed height (H) is shown in Figure 1 below. In general, DP increases with increasing H. When fluidization occurs at the top region of the bed, the curves start to level off. This phenomenon can be explained by theorizing that the combustion of methane occurs here. When methane combusts in air, the molecules of the combustion products expand. As a result, the velocity of the gases increases almost instantaneously. Because the kinetic energy losses predominate at higher gas velocity, DP remains constant throughout the regime.
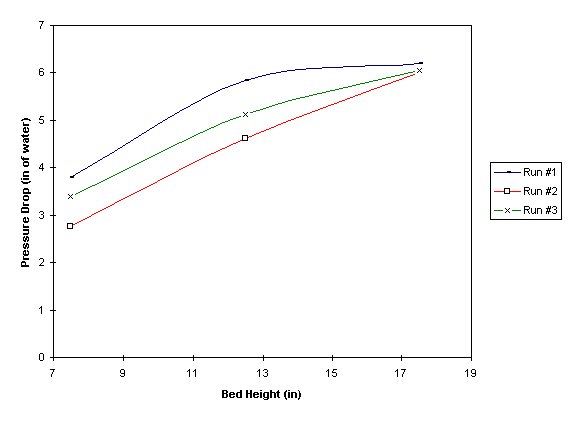
Figure 1: Pressure drop variation along the bed axis
The variation of temperature along the bed axis was also investigated. As shown in Figure 2, temperature increases with increasing bed height. However, on the third run, the curve starts to level off when the bed temperature reaches to about 1300 oF. This phenomenon occurred because a piece of plastic cup was thrown inside the bed to increase the bed temperature to the ignition temperature of methane. As a result, the combustion zone was moved down to somewhere in the middle of the bed. When this happened, the temperature from the middle to the top region of the bed was kept constant at the maximum value.
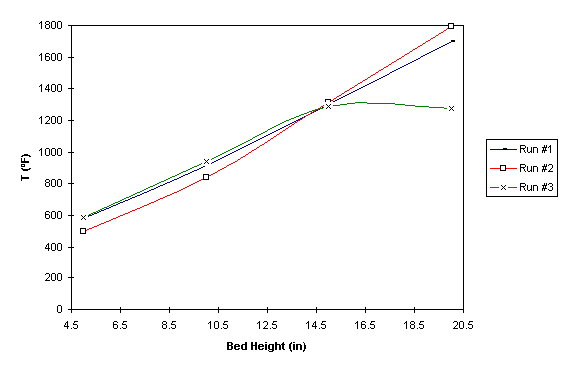
Figure 2: Temperature variation along the bed axis
For the steady state combustion, the flue gas composition was investigated by varying the air-to-fuel ratio. Based on the gas chromatograph (GC) printouts, the true response value (TRV) can be calculated.
TRV = Area % * Response Factor (4)
Where area % can be found from the GC printouts and response factors for all flue gas species are listed in Appendix B. The composition (weight %) of species n in the flue gas stream can then be calculated from Equation 5.
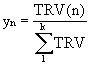 (5)
(5)
Table 3: Composition of the flue gas species
|
Composition |
|||
|
Species |
Run #1 |
Run #2 |
Run #3 |
|
O2 |
0.20 |
0.13 |
0.22 |
|
N2 |
0.74 |
0.74 |
0.69 |
|
CH4 |
- |
- |
0.03 |
|
CO2 |
0.03 |
0.08 |
0.03 |
|
H2O |
0.03 |
0.05 |
0.03 |
As expected, the flue gas containsed some oxygen and nitrogen because excess air was fed into the bed for all three runs. On the first and the second run, no significant amount of methane was detected by the GC. However, on the third run, some amount of methane (about 3 weight percent) was detected in the flue gas. Unfortunately, the reason for the occurrence of incomplete combustion cannot be explained because air was fed more than the stoichiometric amount at all time. Some significant amount of carbon dioxide and water was also detected; their amount was at maximum on the second run. The reason is that more methane was fed into the bed. As a result, more oxygen was consumed in the combustion and, therefore, more carbon dioxide and water were produced.
For the unsteady state combustion, only qualitative observation was made. Several different sample materials were cut into small pieces, having approximately the same size. Two methods of putting these samples into the bed were implemented. First, several pieces of a sample were injected into the bottom of the bed in order to prolong the residence time. As expected, no observation can be made because the sample was completely combusted and converted to gas molecules. Then, several pieces of the same sample were dropped from the top of the bed. Some interesting observations were made; black smoke and fly-ash were seen to come out of the bed. Additionally, the phenomenon inside the bed was observed by holding a mirror on the top of the bed. Some samples burned very rapidly and produced small sparks around them. Others burned slowly and produced large flame. These characteristics depend on both the chemical and physical properties of the materials. For example, polymers burn longer and produce larger flame than tissue papers do. The complete observation of the unsteady state experiment is presented in Table 4 below.
Table 4: Observation of the unsteady state combustion
Characteristics
|
Samples |
Black Smoke |
Fly Ash |
Flame Size |
Speed of Burning |
Other Observations |
|
Styrofoam |
yes |
Small amount (black) |
medium |
fast |
- |
|
Wet Tissue Paper |
no |
no |
small |
slow |
-tiny sparks surrounding tissue first due to water evaporation -middle of paper looked black like burnt paper -slow disintegration of paper |
|
Cork |
yes |
no |
small |
fast |
-catches fire quickly |
|
Plastic |
yes |
large |
large |
fast |
- |
|
Fork |
- |
amount (black) |
- |
- |
- |
|
Plastic |
yes |
large |
large |
fast |
- |
|
Cup |
- |
amount (black) |
- |
- |
- |
|
Rubber (Orange) |
yes |
Medium amount (black) |
very large at first then medium |
Medium speed |
- |
|
Rubber Stopper |
yes |
Medium amount (black) |
very large at first then medium |
medium speed |
- |
|
Wet Newspaper |
no |
Small amount (white/gray) |
small |
very slow |
-tiny sparks surrounding tissue first due to water evaporation -middle of paper looked black like burnt paper |
|
Purple Tape |
yes |
Small amount (black) |
large |
fast |
- |
References